HEARTBEAT Study: A Full DCT Approach
Created: 09.26.2025
Situation
The HEARTBEAT study is a large-scale clinical trial, which aims to explore the potential use of smartwatches in collecting and analyzing biometric data to improve the detection, identification, and understanding of cardiovascular diseases and related conditions.
The HEARTBEAT study, which Samsung showcased at the HLTH 2024 conference in Las Vegas, stands out due to its fully decentralized clinical trial (DCT) approach. The 10,000 adult participants will be remotely monitored over a one-year period, using the Samsung Galaxy Watch 6 to collect biometric data including ECG, heart rate, body composition, and sleep patterns.
Goal
The primary organizational goal for us was to establish a fully decentralized, patient-centric study model that could seamlessly integrate:
-
Continuous smartwatch biomarker data streams
-
Questionnaire responses captured in the HUMADCT platform
-
Clinical evaluations and eligibility confirmations from investigators
-
Secure storage and interoperability between multiple EHR systems
Ultimately, Alcedis’ objective was to deliver an end-to-end DCT workflow that supported real-world data collection at scale, while enabling researchers at Tulane and Samsung to test their scientific hypotheses.
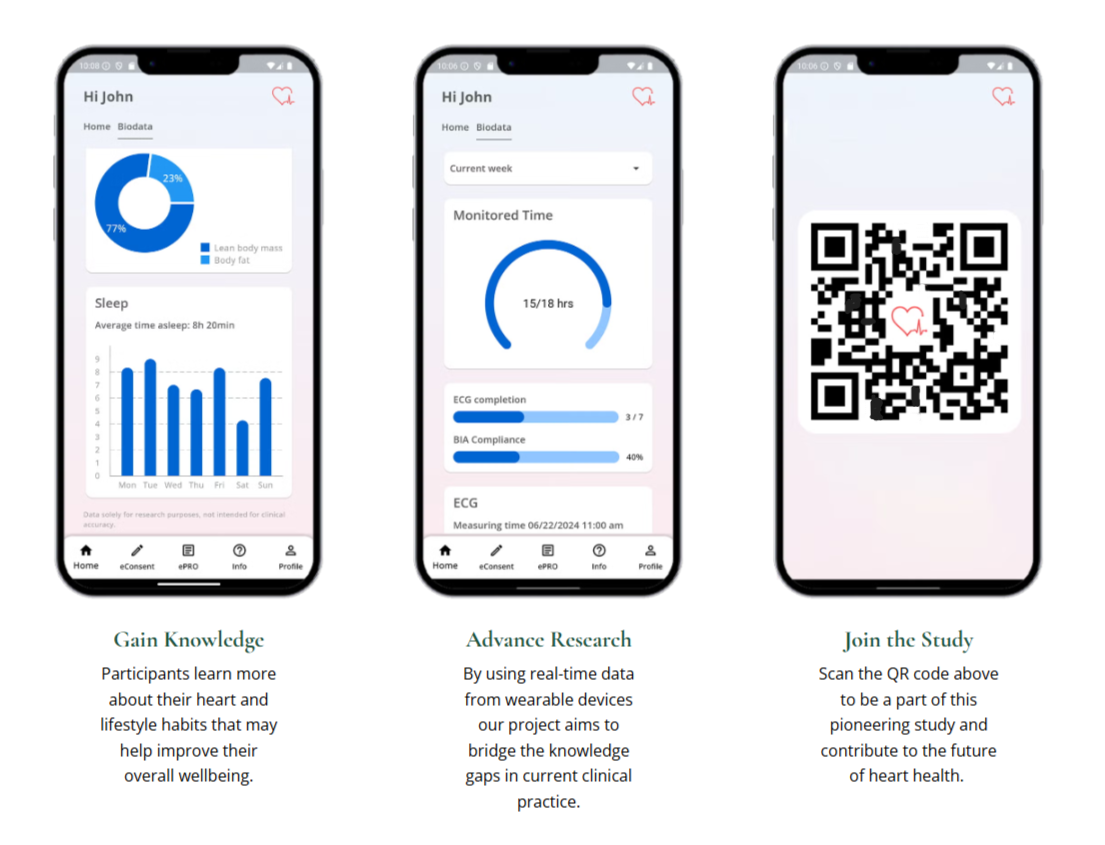
Challenge
The execution of a decentralized study of this scale presented multiple challenges that had to be mastered simultaneously. A key concern lay in the integration of heterogeneous data streams, ranging from smartwatch-derived biometrics to clinical records and digital questionnaires, all of which required harmonization in a single eCRF system. At the same time, regulatory demands in the US necessitated the strict safeguarding of sensitive biometric data in full compliance with HIPAA and FDA standards.
Additionally, the success of the study depended on the ability to not only recruit but engage and train thousands of participants remotely. Ensuring consistent adherence to study procedures over a full twelve-month period, without the structure of regular site visits, posed a significant organizational hurdle. In addition, the study design called for infrastructure capable of handling millions of data points in real time, all while ensuring data integrity and uninterrupted system performance.
Solutions
To meet these challenges, Alcedis implemented a digital infrastructure specifically designed for large-scale decentralized studies. At the center of this setup, the Alcedis eCRF served as the unified system for standardizing and storing all data inputs, ensuring that smartwatch biometrics, EHR records, and app-based questionnaires could be reliably analyzed in combination.
Alcedis applied a multi-layered operational strategy:
-
Unified Data Capture
-
The Alcedis eCRF was designed to harmonize heterogeneous data sources, ensuring that smartwatch-derived biometrics and EHR records could be analyzed side-by-side.
-
-
Seamless App Integration
-
The HUMADCT platform served as the participant interface, providing questionnaires, reminders, and educational content to foster adherence.
-
-
Digital Onboarding & Training
-
Virtual training modules guided subjects in setting up and using their smartwatches, reducing dependency on in-person visits.
-
-
Robust Security & Compliance Framework
-
End-to-end encryption, role-based access, and audit trails ensured compliance with US regulatory standards.
-
-
Adaptive Monitoring
-
Study staff and investigators accessed real-time dashboards to flag dropouts, missing data, or unusual biometric patterns early.
-
Results
From an organizational perspective, the HEARTBEAT study has demonstrated that a fully decentralized approach can be executed at scale in a highly regulated environment. The technical infrastructure was successfully scaled to accommodate 10,000 participants across the US, creating streamlined processes that significantly reduced logistical demands compared to conventional site-based studies.
Continuous data flows from smartwatches, EHR systems, and ePROs were integrated without major losses or duplications, underscoring the robustness of the unified data capture system. The security framework provided assurance that all data management processes complied with HIPAA and FDA requirements.
Early feedback from participants confirmed that digital onboarding and app-based engagement strategies were well received, indicating strong acceptance of the decentralized model among study subjects. Together, these outcomes mark an important step toward validating decentralized methods for longitudinal, non-interventional research at scale.
Conclusion
The HEARTBEAT study demonstrates how a CRO can successfully operationalize a large-scale decentralized clinical trial by combining wearable devices, EHR integration, and digital platforms into a single cohesive framework.
From Alcedis’ perspective, the project showcases:
-
The feasibility of wearable-based clinical research at scale
-
The robustness of decentralized workflows for longitudinal, non-interventional studies
-
The potential of real-world, patient-driven data to accelerate cardiovascular research
This case underlines that future-ready CROs must master not only trial execution but also digital infrastructure, data interoperability, and patient engagement strategies to enable the next era of evidence generation.
A report by WWL-TV:
https://www.youtube.com/watch?v=feTPByzSuQk

