FINE-REAL: Innovational EHR2EDC Pilot
Created: 09.29.2025
Situation
The FINE-REAL study, a global post-authorization safety study (PASS) for a newly approved non-steroidal, selective mineralocorticoid receptor (MR) antagonist, was designed to run across more than 20 countries, enrolling over 4,500 participants in more than 250 sites. As part of this ambitious project, several technical pilot initiatives were launched to explore how innovative approaches could support efficiency, data quality, and long-term feasibility in such a complex research environment. Among these pilots, one of the most groundbreaking was the evaluation of an automated transfer of data from electronic health records (EHR) to electronic data capture (EDC) systems—an approach often referred to as EHR2EDC.
Goal
Our objective was not simply to run another observational study. Instead, we aimed to establish a forward-looking model that could demonstrate how routine healthcare data could be harnessed directly for clinical research purposes. By reducing the manual burden on physicians, ensuring higher data integrity, and decreasing overall study costs, we wanted to show that EHR2EDC could bridge the gap between daily clinical practice and regulatory-grade research data.
Challenge
The main difficulty lay in the heterogeneity of practice management systems and the absence of universal data standards across sites. Frequent additions to our DAT system were required, alongside the development and implementation of a new data-extraction tool specifically tailored to Germany, acting as the pilot country for this approach. Each of these steps demanded careful alignment with regulatory requirements, seamless technical integration, and robust data protection according to the European General Data Protection Regulation (GDPR). At the same time, the sponsor expected rapid progress and clear results, which meant we needed to move quickly without compromising quality.
Solutions
We addressed this challenge through a highly collaborative and agile approach. By maintaining a close dialogue with the sponsor and establishing short internal decision paths, we were able to design, test, and refine new tools while the study was ongoing. Together with our partners, we implemented a technical framework that allowed the automated extraction of selected variables from EHRs and their secure transfer into our validated EDC platform. The model was based on an end-to-end pipeline: data were harmonized and anonymized at the practice level, transferred through an intermediary layer, and imported directly into the study’s electronic case report forms (eCRFs). This eliminated the need for redundant manual data entry and ensured consistency across systems.
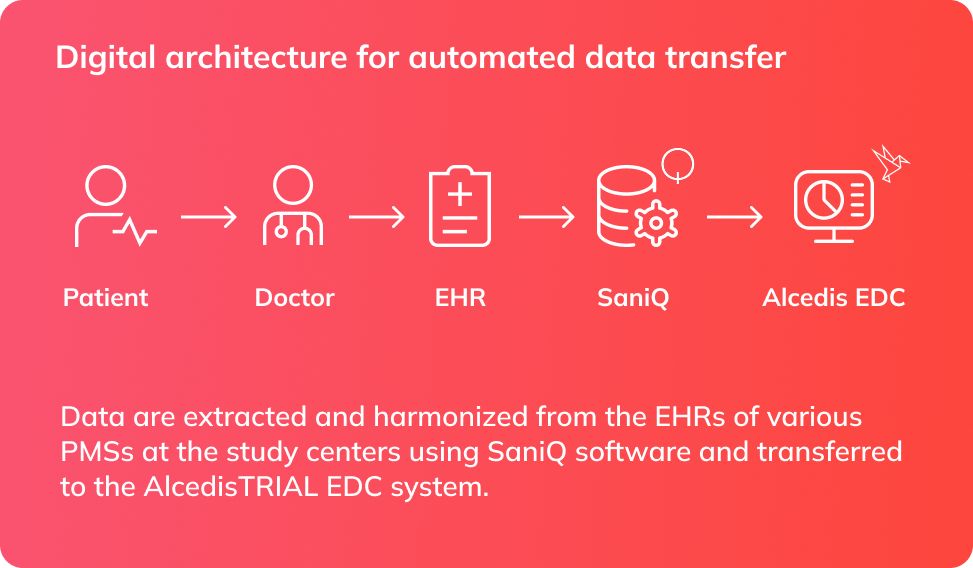
Results
The EHR2EDC pilot proved that automated transfer of clinical routine data is feasible within the German healthcare system. In practice, nearly one quarter of all variables in the study’s eCRFs could be populated automatically, with more than one third of eligible data fields transferred without manual intervention. The direct data extraction approach saved physicians significant time, reduced the likelihood of transcription errors, and lowered the overall costs associated with data collection. Beyond the immediate benefits, the pilot delivered reusable tools and valuable insights for future studies, creating a foundation for long-term efficiency gains and strengthening our partnership with the sponsor.
Conclusion
The FINE-REAL EHR2EDC pilot demonstrated how technical innovation can transform the interface between healthcare practice and clinical research. By proving that automated data flows are possible in a real-world PASS, we not only addressed immediate project needs but also contributed to shaping a future in which routine clinical data can reliably support regulatory decision-making. This case underlines that with the right mix of collaboration, agility, and technical expertise, the gap between clinical care and research can be closed—leading to faster, more reliable, and more cost-effective studies.
Joint publication
The concept was published together with the sponsor team in a peer-reviewed journal.

