Our passion is research
Digitalized Clinical Research
We examine, we advise, we plan and we fully deliver on a global scale from phase I to drug approval, beyond market access into real world evidence - with over 30 years of experience.
Alcedis Services
Our DNA is code
Alcedis IT-Solutions
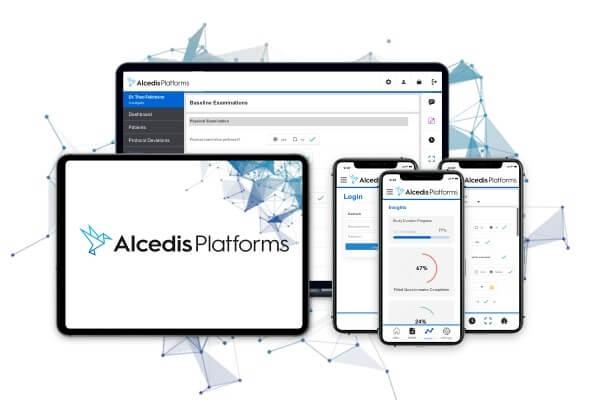
Alcedis Platforms™ is the powerful technology for multi-channel data integration in clinical studies - with customized eCRFs, mobile applications, big data interfaces and much more to explore.
Discover Possibilities
Realizing and performing biotech research
Leverage Alcedis' extensive experience and expertise in operational conduct of clinical trials within the biotechnology sector
Learn morePROCEDURE
How we make a difference
It all starts with our clients goals and visions. We find scientific objectives, optimum measures and suitable technologies to create a significant difference before the study conduct even starts.
Our Process
Blog
Learn more about Alcedis and the industry
Clinical data management: Efficiency and Security in Clinical Trials
DCT: Wie dermato-onkologische Studien von Digitalisierung profitieren
Let's create an impact. Together.
Create a powerful environment for research and contact us today.
Contact Experts