Our passion is research
Digitalized Clinical Research
We examine, we advise, we plan and we fully deliver on a global scale from phase I to drug approval, beyond market access into real world evidence - with over 30 years of experience.
Alcedis Services
Our DNA is code
Alcedis IT-Solutions
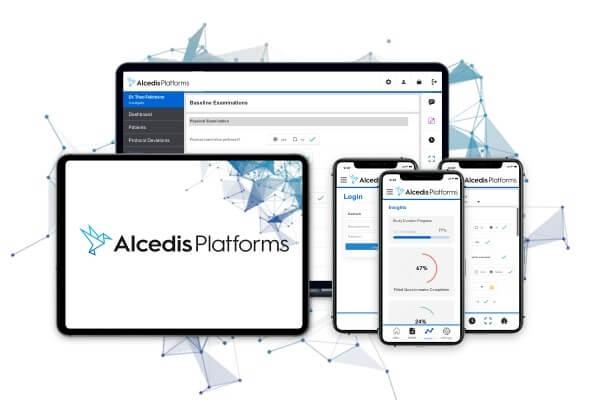
Alcedis Platforms™ is the powerful technology for multi-channel data integration in clinical studies - with customized eCRFs, mobile applications, big data interfaces and much more to explore.
Discover Possibilities
The future of oncology research
By using state-of-the-art technologies such as patient-centered apps, ePRO/eCOA, wearables or telemedicine, we offer you the perfect combination of comprehensive services and innovative technical solutions as a full-service CRO.
PROCEDURE
How we make a difference
It all starts with our clients goals and visions. We find scientific objectives, optimum measures and suitable technologies to create a significant difference before the study conduct even starts.
Our Process
Blog
Learn more about Alcedis and the industry
Partnerships with CROs in Clinical Trials: A Key Success Factor for Pharma and Biotech Companies
AI in Clinical Trials: Training Machine Learning Algorithms
Let's create an impact. Together.
Create a powerful environment for research and contact us today.
Contact Experts