EUMelaReg: How Alcedis Supports the Development of a Melanoma Registry
Created: 09.26.2025
Background
Skin cancer is among the most common types of cancer. According to the German Cancer Society, more than 3,700 people died from it in 2017. The most common cause is malignant melanoma, also known as black skin cancer. It is the most dangerous form of the disease.
In recent years, diagnostic and treatment options for skin cancer have advanced further. In addition, national cancer registries show what impact treatment methods have on the course of the disease in patients. While these registries primarily collect and analyze data within their respective countries, the European Melanoma Registry Project (EUMelaReg) has been comparing patient information across countries since 2018 to combat skin cancer.
Goals
The international melanoma registry EUMelaReg was created with one goal: the collection and evaluation of real-world data on the available diagnostic and treatment patterns of patients with malignant melanoma and other types of skin cancer on a European level. The establishment of the registry enables the monitoring of melanoma care across Europe. For this purpose, epidemiological study results, clinical treatment pathways, and health economic aspects of the respective countries are jointly analyzed.
The following tasks are in focus:
-
Creation and maintenance of a central registry for melanomas and defined subtypes
-
Data analyses to improve diagnoses and to develop new therapies
-
Comparison of medical histories, treatment courses, and drug usage
-
Overview of country-specific drug approvals as well as treatment methods and their impact on side effects or survival rates
Challenges
A project of this scale is demanding, and integrating different countries with their individual datasets is complex. For establishing data transmission into the international melanoma registry, each country must overcome different hurdles. Examples include:
-
Coordination Across Borders: Integrating data from different countries with varying healthcare systems and registry standards.
-
Technical Integration: Ensuring secure, automated data transmission while accommodating countries without existing melanoma registries.
-
The implementation of new systems on site can be challenging and delay automated data exchange; until then, data must be transferred manually.
-
Patient consent in the respective countries regarding the transfer of patient data to EUMelaReg must be clarified and, where necessary, approved by national ethics committees.
-
Data Harmonization: Standardizing and mapping diverse data structures from national registries to a unified format.
In countries with their own local melanoma registry, the differing database structures present further challenges for the team. A standardized integration of these structures implies extensive preparation and follow-up work.
The so-called “mapping” compares data fields of a country’s database with those of EUMelaReg and aligns them accordingly. This includes, among other things, information from medical history forms, such as pre-existing conditions, which are often provided in the respective national language.
Solution
From the very beginning, the CRO Alcedis supported the EUMelaReg project with its IT infrastructure and many years of big data expertise. Alcedis was responsible for the technical setup of the registry and for transferring the data from the various partner countries.
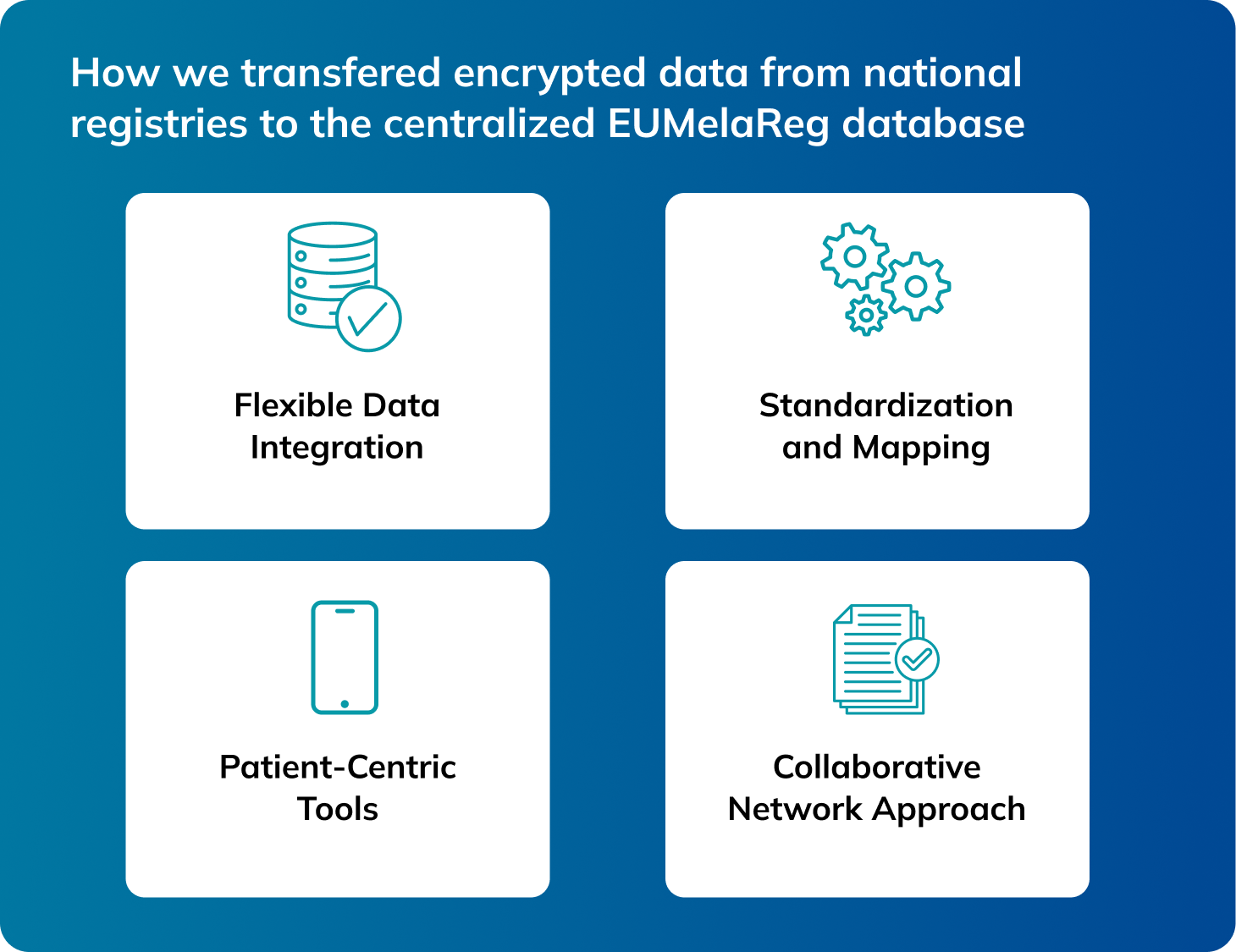
We facilitated the secure transfer of encrypted data from national registries to the centralized EUMelaReg database using XML and other standardized formats. For countries without existing systems, Alcedis created national melanoma registries and ensured their seamless integration into the EUMelaReg framework. Additionally, they worked closely with local doctors, data managers, and national ethics committees to ensure compliance and facilitate data sharing.
-
Flexible Data Integration
-
For countries with existing national registries, bespoke interfaces and XML-based transfer protocols were implemented to securely import data into EUMelaReg.
-
For countries without registries, Alcedis built national-level databases, enabling hospitals and melanoma centers to enter and maintain data locally.
-
-
Standardization and Mapping
-
A structured data mapping process aligned local variables with the EUMelaReg standard, covering epidemiological, clinical, and health-economic aspects.
-
Language barriers and inconsistent coding systems were resolved through harmonization tools and dedicated workflows.
-
-
Patient-Centric Tools
-
In sub-studies, patients were given access to mobile apps that allowed them to report outcomes electronically (eCOA), directly feeding into the registry.
-
-
Collaborative Network Approach
-
Continuous communication with national coordinators ensured that missing data points could be identified, discussed, and—where feasible—incorporated into future documentation.
-
Annual reports for sponsors combined comprehensive cross-country data analysis with detailed subgroup insights.
-
Results
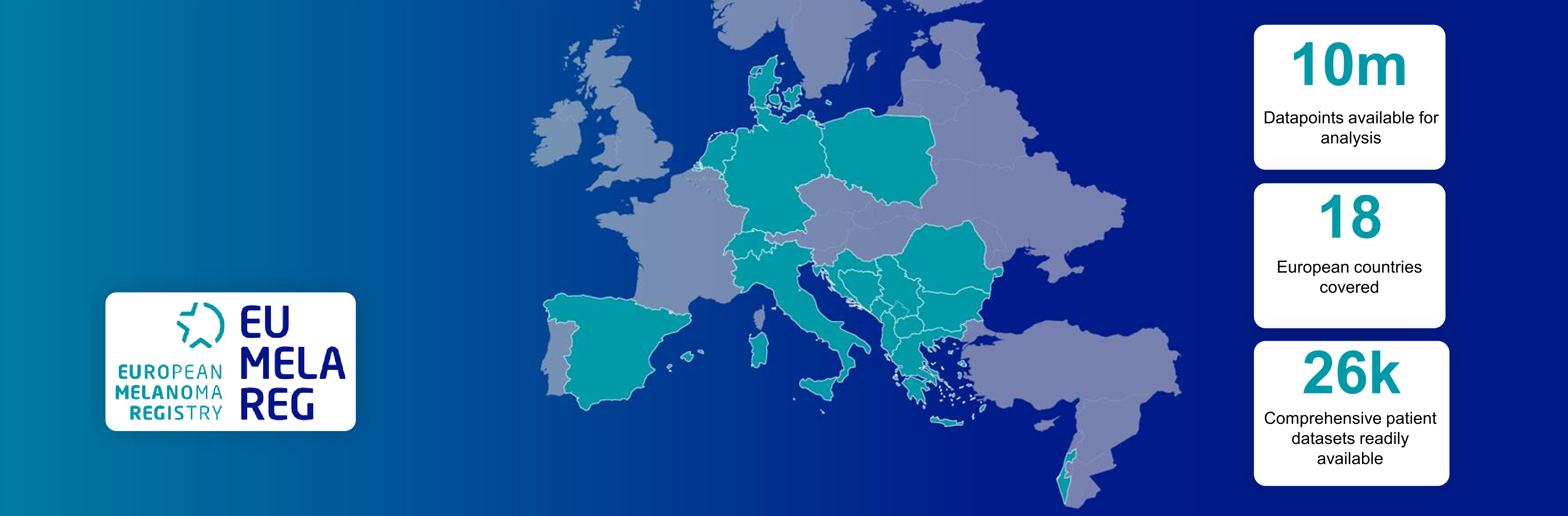
The EUMelaReg project has achieved significant milestones. The registry cooperation founded in Germany started in 2018 with the countries Croatia, Serbia, Bosnia and Herzegovina, Spain, Denmark, and the Netherlands. With now 18 partner countries and more than 26,000 patient datasets, EUMelaReg is one of the fastest-growing melanoma databases in the world.
High-quality, standardized data collection has improved the understanding of melanoma treatment and outcomes across Europe. The registry supports epidemiological research, clinical treatment analysis, and health economic evaluations, leading to better-informed healthcare decisions and policy-making. Continuous addition of new countries and datasets enhances the registry’s value, contributing to more precise and comprehensive insights into melanoma and other skin cancers.