Alcedis eCRF
Verschlanken Sie die Datenerfassung am Point of Experience und spiegeln Sie alle relevanten Prozesse des Daten-, Sicherheits- und Standortmanagements, des klinischen Betriebs und der Logistik in einem einzigen System wider.
Datenqualität
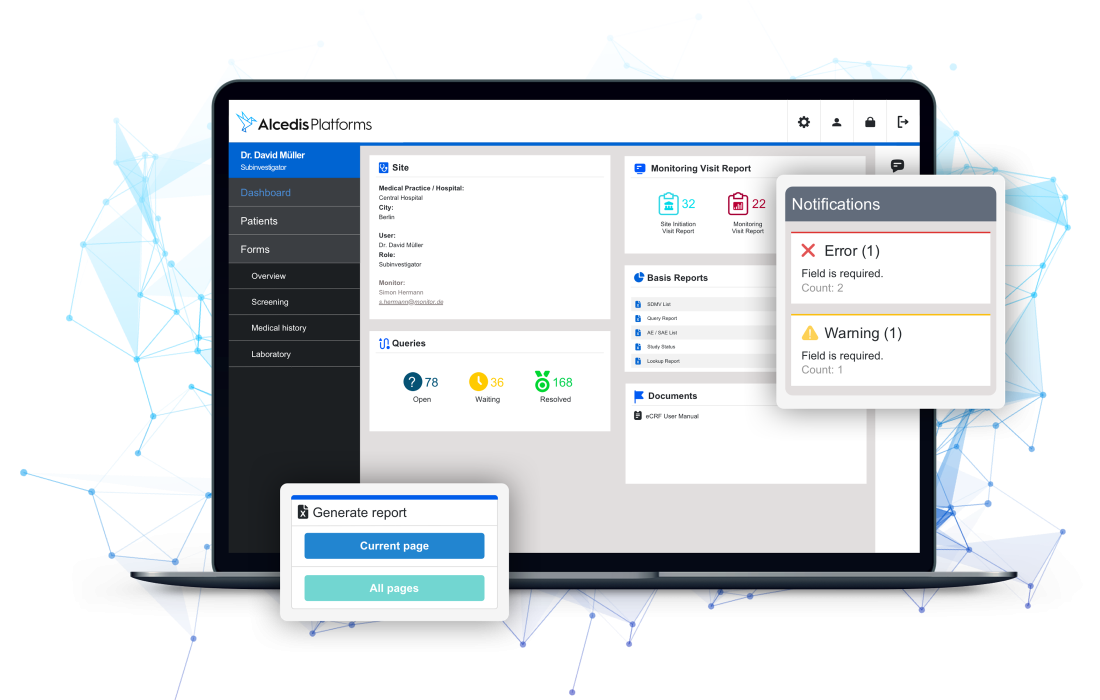
Mit intelligenter und automatisierter Echtzeit-Datenvalidierung reduzieren wir effizient bis zu 80 % des manuellen Datenbereinigungsaufwands. Patientendaten sind in modernen und übersichtlichen Schnittstellen für relevante Power-User wie CRAs, Daten- oder Projektmanager einfach zu überwachen. Alle Aktivitäten sind in einem Audit-Trail und einer rollenbasierten Zugriffskontrolle vollständig transparent.
- Echtzeit Datenvalidierung
- Einfaches Daten-Monitoring

Überwachung
Benutzeradaptive Dashboard-Instrumente liefern wertvolle Informationen zur Übersicht. Status der Website-Aktivitäten, Patientenrekrutierung, Vollständigkeit der Dokumentation, erreichte Meilensteine und vieles mehr können individuell mit einem Lesezeichen versehen oder über eine semantisch-elastische Suche dynamisch gefunden werden. Zusätzliche Warn- und Prognosesysteme stellen sicher, dass die Studie auf dem richtigen Weg ist.
- In-App Bookmarking, um die wichtigsten Informationen immer im Blick zu behalten.
- Elastic Search, um schnell Antworten auf wichtige Fragen zu finden.
Reporting
Alcedis eCRFs werden mit einer leistungsstarken Reporting-Toolbox geliefert, die individuell angepasst und mühelos konfiguriert werden kann. Sammeln Sie Echtzeit-Reportings für risikobasierte Studiendurchführung, Zwischenanalysen und weitere Statistiken zu jeder Zeit und in Multi-Format-Visualisierung.
- Konfigurierbare und leistungsstarke Toolbox für Ihr Reporting
- Push-Benachrichtigungen, um keine relevanten Study Events zu verpassen

Analyse
Erfahren Sie mehr über Ihre Studie und Ihre Patienten.
Ihr eCRF bietet Ihnen überlegene, KI-basierte Analytics-Funktionen, um tiefer in Ihre Daten blicken zu können.
Decken Sie Anomalien auf, erfassen Sie versteckte Adverse Events und vieles mehr.
- KI-basierte Analyse zur Identifizierung und Vorhersage versteckter Safety Events
- Datenerkennungsmodelle, um kritische und entscheidende fehlende Teile aufzudecken

Starten Sie mit Alcedis Platforms™!
Für mehr und hochwertigere Daten. Für erfolgreiche Pharmazie. Für die Forschung.
Beratung anfordern